A Note from the President
I’m proud to start out this newsletter by announcing that as of July 20th, TransPharm Preclinical Solutions has once again received a recommendation for accreditation from the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International.
AAALAC accreditation is evaluated every three years. Programs constantly change, as do the regulations for the use of animals in research. AAALAC’s mission is to ensure that all of these changes are enforced and that the Program Description for our facility is accurate, complete, and in adherence. The continued success of our program is directly related to those individuals on our Institutional Animal Care and Use Committee (IACUC). I would like to personally thank the following individuals:
- Libby Ziemke, IACUC administrator
- Marci Peek, Chair
- Dr. Megan Nowland, Attending Veterinarian and Alternate Chair
- Joanne Zammit, Scientist
- Dr. Santiago Lopez, Scientist
- Dan Wymer, Non-Scientist
- Dr. Jim Mobley, Alternate scientist
Adhering to these global standards is what led to our success three years ago, and this continued recommendation for accreditation bodes well for our continued success. To learn more about AAALAC International please visit www.aaalac.org.
Towards our commitment to high-quality research, four individuals here at TransPharm have recently received certification through the American Association of Laboratory Animal Science (AALAS). AALAS offers nationwide board exams after certain criteria are met. I would like to congratulate:
- Heidi Richardson, ALAT level
- Marci Peek, LAT level
- Libby Ziemke, LATG level
- Lisa Lazaroff, LATG level
To learn more about AALAS please visit www.aalas.org.
Daniel Ross
President and CEO
Clostridium difficile murine model
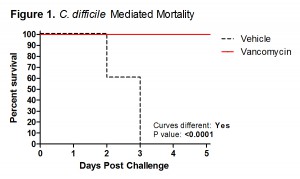
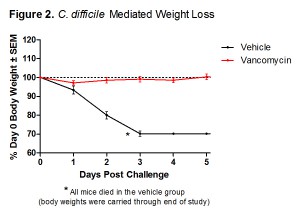
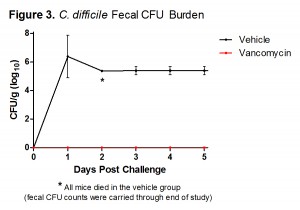
In an effort to provide our clients with the most robust and effective murine models of infection, TransPharm has adopted a new bacterial spore preparation for our C. difficile-associated disease model. This improved protocol has been thoroughly validated and rigorously compared to our previous procedure. It has surpassed TransPharm’s high standard of scientific excellence (Figures 1-3), much to the satisfaction of our valued clients with whom we’ve had the pleasure of collaborating.
So, if your group is in the market for a murine model of C. difficile-associated disease (or any other infectious disease model), there’s no better time to talk with us! Don’t forget – PK studies are also available here at TransPharm.
Dr. Santiago R. Lopez
Director of Research
ICAAC 2012
TransPharm will attend the 2012 ICAAC conference in San Francisco taking place September 9th – 12th. There have been many positive changes within TransPharm since the 2011 ICAAC in Chicago, including new animal models, management, and additions to our scientific team. Our new Director of Research, Dr. Santiago Lopez, will be joining us in San Francisco. Please stop by booth #1014 and welcome him to his first ICAAC.
As always, please feel free to contact us:
Through our UPDATED website, www.transpharmsite.com
Our toll free INFECT line, 1-888-88-INFECT
Or dial me direct, 1-517-536-8210
Check us out on Facebook