Winter 2019 Newsletter
Winter 2019 Newsletter
A Note from the President
The new year is off to a chilly start in Michigan! Polar Vortex 2019 brought record low temperatures, several inches of snow, and lots of slippery ice. Schools, businesses, and even the state government closed due to the weather, but TransPharm only shut down for 4 hours during the two weeks of Arctic conditions. We are committed to being available for our clients regardless of Mother Nature’s plans!
Thinking back to warmer days, 2018 was another record year for TransPharm. With 20% more business than in 2017, we had our highest annual revenue to date. The team and our families celebrated our accomplishments and the holiday season at a party in December.
Daniel Ross
President & CEO

In the Lab
A Two-Hit Model of Sepsis
TransPharm recently validated a “two-hit” model of sepsis in which mice undergo cecal ligation and puncture (CLP) followed by intranasal instillation with bacteria to initiate a pulmonary infection.
Although sepsis is a major cause of mortality in hospital patients, death often results from the synergistic effect of a concurrent nosocomial infection rather than the initial septic event on its own. Data indicates that primary sublethal sepsis weakens the immune system and increases susceptibility to secondary infection and death. Our two-hit model is clinically relevant as it reflects the increased yet delayed mortality from a secondary infection, such as pneumonia, after sepsis. Further, the extended duration of infection allows for the evaluation of therapeutics targeting specific stages of the immune response.
TransPharm has validated this model using Pseudomonas aeruginosa, although published literature has also demonstrated the effect with Staphylococcus aureus and Streptococcus pneumoniae. Validations with these and other pathogens are available upon request. Contact us to request additional information or a free, no-obligation quote.
Intratracheal Inoculation and Dosing
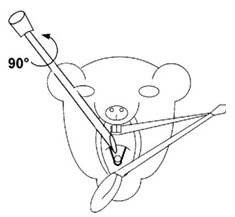
Our Institutional Animal Care and Use Committee (IACUC) recently approved intratracheal (IT) administration as a new route of inoculating and dosing mice. Intratracheal administration delivers infections and compounds directly to the lungs, while intranasal (IN) administration serves as an indirect delivery method via inhalation.
To perform intratracheal administration of a challenge, test article, or comparator drug, mice are anesthetized and forceps are used to gently retract the tongue. Then, bacteria or compound are delivered into the trachea using an illuminated catheter. The figure (right) illustrates this technique and the correct position of the catheter.
Contact us today for more information or to request a free, no-obligation quote for your study.
Santiago R. Lopez, Ph.D.
Chief Scientific Officer