Systemic Infection – Fungemia
Systemic Infection – Fungemia
Fungemia is defined as the presence of fungi in the bloodstream, which is normally a sterile environment. As these fungi multiply, a potentially life-threatening infection called sepsis can develop. These conditions arise from fungi originating elsewhere in the body. Our fungemia studies allow for in vivo screening of antimicrobial activity to rapidly identify the most promising therapies.
Procedure
We have established a fungemia model in mice (validation in rats is available upon request) in which animals are challenged with a fungus via intravenous (IV) injection in the lateral tail vein. Test articles may be administered via oral gavage (PO) or subcutaneous (SC), intravenous (IV), or intraperitoneal (IP) injection. Each study parameter (animal strain, pathogen, comparator(s), and dosing schedule) can be customized to meet client needs.
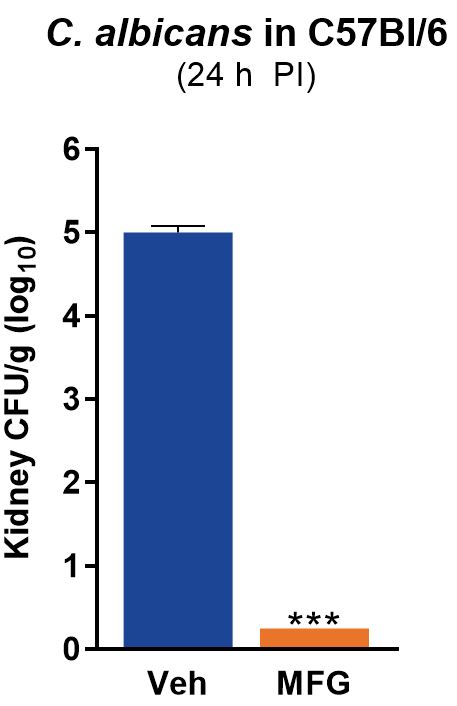
Endpoints for this study include mortality tracking and CFU burden in harvested organs and/or blood. Pharmacokinetic profiling, blood chemistry, tissue harvests, and histology are available upon request.
Sample Data
Pathogens
TransPharm has validated a fungemia model using the following pathogens:
- Aspergillus fumigatus
- ATCC 3073
- Candida albicans
- ATCC SC5314
Additional validations are available upon request.